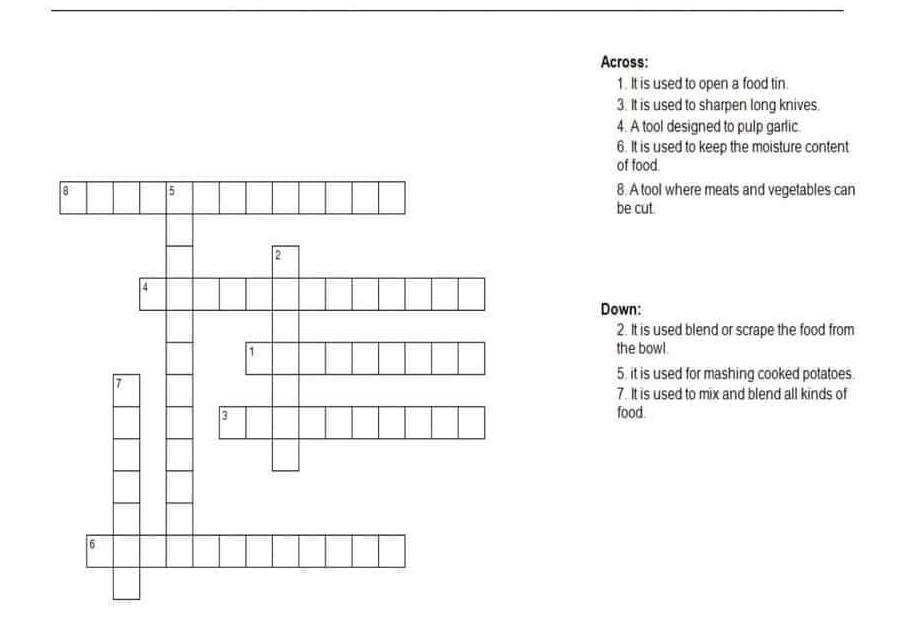
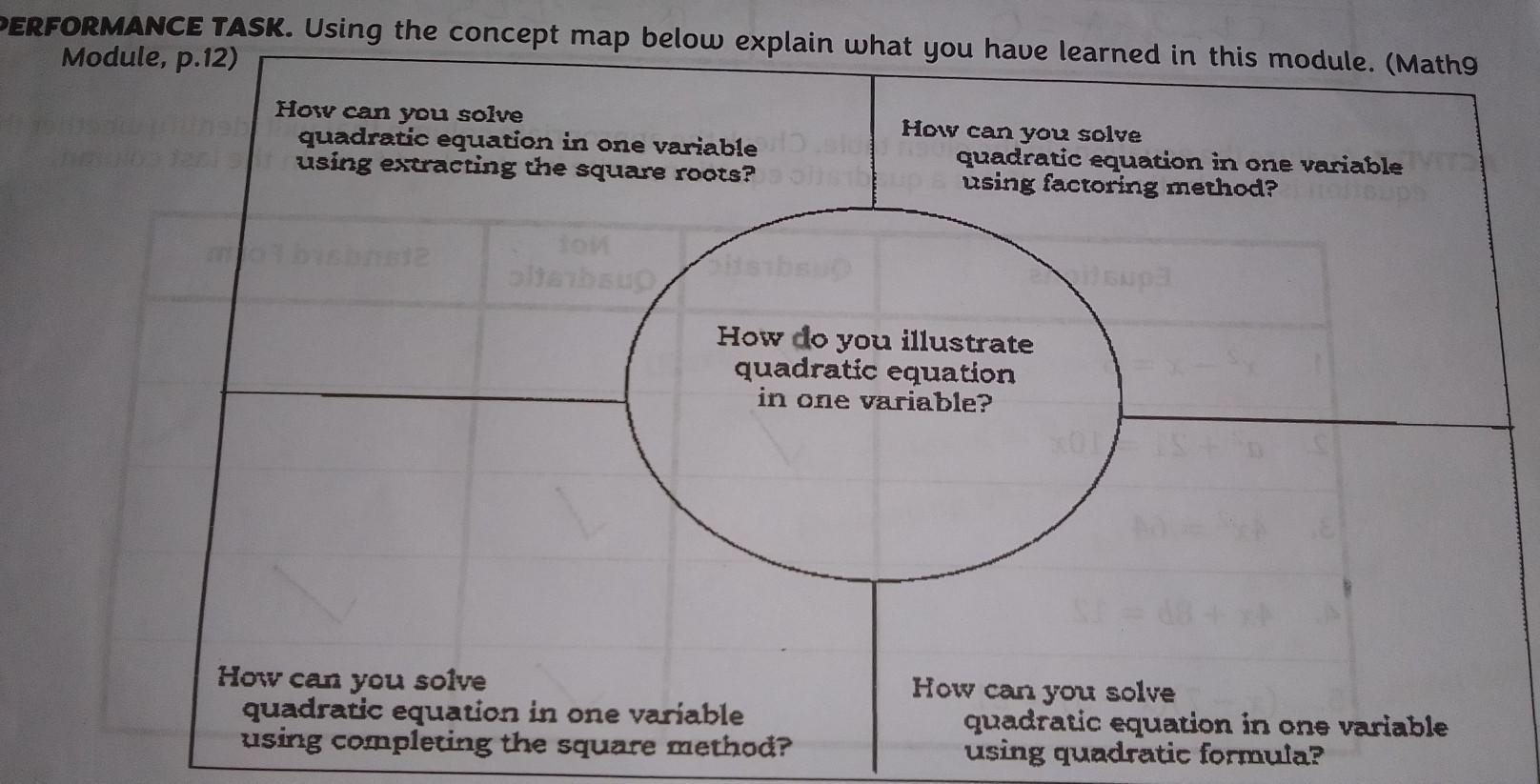
Arrange the word (SIOPOTIPRON) (MASTYSETICBODUT)
-
Subject:
Integrated Science -
Author:
rose13 -
Created:
1 year ago
Answers 1
Answer:
Proposition
Sa 2 Diko alam
-
Author:
dakotahyov
-
Rate an answer:
10
Do you know the answer? Add it here!
Choose a language and a region
How much to ban the user?
1 hour
1 day
100 years